Abstract
Background: Myeloablative conditioning can be given safely to older patients by simply administering busulfan over a longer period (fractionated busulfan regimen) than the standard four-day regimen (Popat et al Lancet Haematology 2018). This longer duration of conditioning regimen allows addition of targeted agents like Venetoclax, which may be synergistic with conditioning chemotherapy and may further improve disease control with this regimen. We therefore added Venetoclax to our ongoing prospective clinical trial with f-Bu-Flu-Cladribine conditioning (NCT02250937). After enrolling 83 patients, the study was amended and venetoclax was added for the next 33 patients. Here we report the safety and preliminary efficacy of venetoclax and fractionated busulfan regimen.
Methods: Between 2/2019 and 3/2021, 33 patients with AML (n=21) or MDS (n=10) up to 70 years of age with adequate organ function and 8/8-HLA matched related or unrelated donor were enrolled on a prospective trial. The conditioning regimen was f-Bu to target an area under the concentration vs time curve (AUC) of 20,000 ± 12% μmol.min given over a period of 2-3 weeks. The first two doses of busulfan (80 mg/m2 IV each) were administered either consecutively (days -13 and -12) or with further fractionation, one week apart (days -20 and -13) on outpatient basis. Then, inpatient fludarabine 10 mg/m 2, and cladribine 10 mg/m 2 were given followed by Bu on days -6 to -3. Venetoclax 400mg daily was given from day -22 to -3. Azoles were avoided during this period. GVHD prophylaxis was PTCy 50mg/kg on days 3 and 4 and tacrolimus from day 5.
Results: The median age was 59 years (range, 23-69); High or very high disease risk index was present in 21%; Comorbidity index score of >3 was present in 45%; Donor was a sibling in 39%; and peripheral blood stem cells was the graft source in 100%.
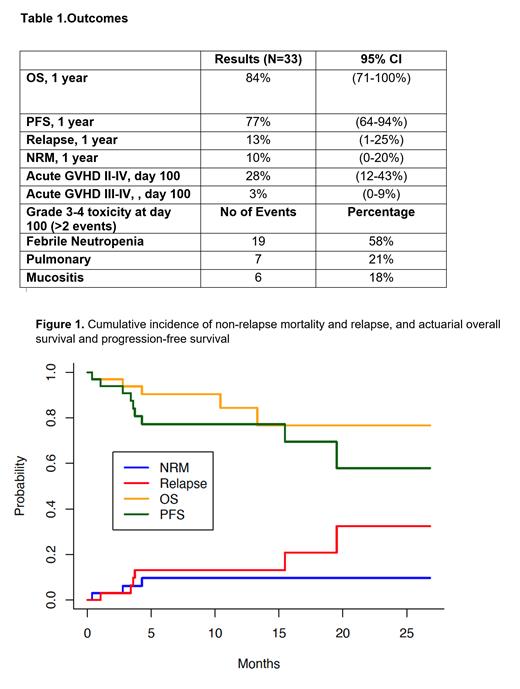
The median follow up was 8 months. At 1-year, overall survival was 84% (95% confidence interval, 71-100), progression-free survival 77% (64-94), relapse 13% (1-25), and non-relapse mortality 10% (0-20) [Table 1, Figure 1]. Incidence of acute GVHD grade 2-4 was 28% (12-43) and grade 3-4 acute GVHD was 3% (0-9) at day 100. All patients engrafted. The median time to neutrophil engraftment was 15 days (13 -19) and median time to platelet engraftment was 23 days (11-85). Full donor chimerism at day 30 was noted in 76%. Common grade 3 or 4 toxicity were neutropenic fever (58%), mucositis (18%) and pulmonary toxicity in 21%.
Conclusion: Venetoclax can be safely added to the fractionated busulfan regimen. Early data on efficacy appear promising.
Popat: Bayer: Research Funding; Abbvie: Research Funding; Novartis: Research Funding; Incyte: Research Funding. Mehta: CSLBehring: Research Funding; Syndax: Research Funding; Incyte: Research Funding; Kadmon: Research Funding. Hosing: Nkarta Therapeutics: Membership on an entity's Board of Directors or advisory committees. Gulbis: EUSA Pharma: Other: Advisory board participation. Rezvani: AvengeBio: Other: Scientific Advisory Board ; Pharmacyclics: Other: Educational grant, Research Funding; GemoAb: Other: Scientific Advisory Board ; Navan Technologies: Other: Scientific Advisory Board; Bayer: Other: Scientific Advisory Board ; Caribou: Other: Scientific Advisory Board; Takeda: Other: License agreement and research agreement, Patents & Royalties; Virogin: Other: Scientific Advisory Board ; GSK: Other: Scientific Advisory Board ; Affimed: Other: License agreement and research agreement; education grant, Patents & Royalties, Research Funding. Qazilbash: Bristol-Myers Squibb: Other: Advisory Board; Oncopeptides: Other: Advisory Board; Amgen: Research Funding; Angiocrine: Research Funding; NexImmune: Research Funding; Biolline: Research Funding; Janssen: Research Funding. Kadia: Cure: Speakers Bureau; Novartis: Consultancy; Dalichi Sankyo: Consultancy; Cellonkos: Other; Ascentage: Other; Genfleet: Other; Sanofi-Aventis: Consultancy; Genentech: Consultancy, Other: Grant/research support; Astellas: Other; Liberum: Consultancy; BMS: Other: Grant/research support; Amgen: Other: Grant/research support; Aglos: Consultancy; Pfizer: Consultancy, Other; AstraZeneca: Other; AbbVie: Consultancy, Other: Grant/research support; Pulmotech: Other; Jazz: Consultancy. Konopleva: Calithera: Other: grant support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Ascentage: Other: grant support, Research Funding; Cellectis: Other: grant support; Ablynx: Other: grant support, Research Funding; Agios: Other: grant support, Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; AstraZeneca: Other: grant support, Research Funding; Stemline Therapeutics: Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Forty Seven: Other: grant support, Research Funding; Sanofi: Other: grant support, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; KisoJi: Research Funding. Shpall: Axio: Consultancy; Magenta: Consultancy; Novartis: Consultancy; Bayer HealthCare Pharmaceuticals: Honoraria; Adaptimmune: Consultancy; Navan: Consultancy; Takeda: Patents & Royalties; Novartis: Honoraria; Affimed: Patents & Royalties; Magenta: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal